|
|
Date: July 30, 2013
Company: Stratatech Corporation of Madison, WI
Contract amount: The initial contract is for two years and approaching $9.9 million. The contract can be extended for up to a total of five years and up to $47.2 million.
About the contract:
The contract is part of a broader effort by ASPR’s Biomedical Advanced Research and Development Authority (BARDA) to develop new medical products to protect health and safety during a radiological or nuclear emergency, such as the detonation of a nuclear device. |
In a radiological or nuclear emergency, people are likely to have combined injuries involving trauma, radiation, and thermal burns. The detonation of a nuclear bomb or improvised nuclear device produces intense heat, potentially resulting in severe burns that can cover large portions of a patient’s body. Such thermal burns typically require specialized treatment, including surgical intervention to remove burned tissue.
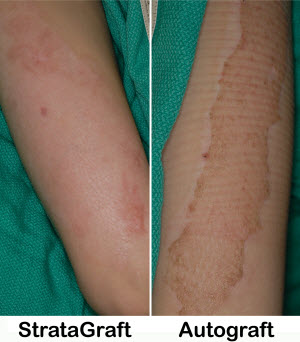
To provide temporary coverage for the resulting wound bed, cadaver skin is often applied to promote healing, reduce fluid loss, and prevent infection. In severe cases where the skin layers are fully damaged, the wounds require skin grafts taken from the patient’s own body. Stratatech Corporation’s technology, StrataGraft, potentially could provide more effective coverage for severe burn wounds than these more traditional methods.
StrataGraft was developed from a special line of human cells discovered at the University of Wisconsin, Madison. This living cell-based tissue has characteristics similar to human skin and can be grown indefinitely in the laboratory.
Because, like healthy human skin, StrataGraft includes an epidermal and dermal layer of cells, the product could be a viable skin substitute where both layers of the patient’s skin have been damaged by the burn. This resorbable tissue may be easily sutured and could remain intact, functioning as a critical barrier during the wound healing process.
StrataGraft has been tested on burn patients in Phase I and Phase II clinical trials, and under this contract Stratatech will enhance the product to extend shelf-life, adopt a larger, more user-friendly shape, and simplify the way the cells are grown.
If the contract is extended, the company will test this enhanced version in clinical trials to demonstrate its safety and efficacy, including testing for pediatric burn patients. Additional work also would focus on improving the manufacture of the tissue to make it more cost competitive.
This contract is part of a broader BARDA effort to develop products for thermal burn injuries and for use in combined injuries likely in mass casualty situations. This line of product development is the latest in BARDA’s portfolio of advanced research and development contracts for emergency medical countermeasures to treat acute radiation syndrome resulting from bone marrow, lung, gastrointestinal and skin injuries caused by damaging levels of radiation.
Many of the products that BARDA is developing may find uses outside of emergency medicine, especially in the case of severe thermal burns. The development of such medical products improves the nation’s preparedness by increasing the number of products available for emergencies. With multiple uses the market for these emergency products can be sustained because the products also can be used in day-to-day medical situations.
